Scientists uncovered really nan brain’s furniture nucleus of nan stria terminalis acts arsenic a maestro switchboard, merging reward and request signals to power eating, a find that could guideline caller treatments for obesity and illness-related weight loss.
Study: A encephalon halfway that controls consummatory responses
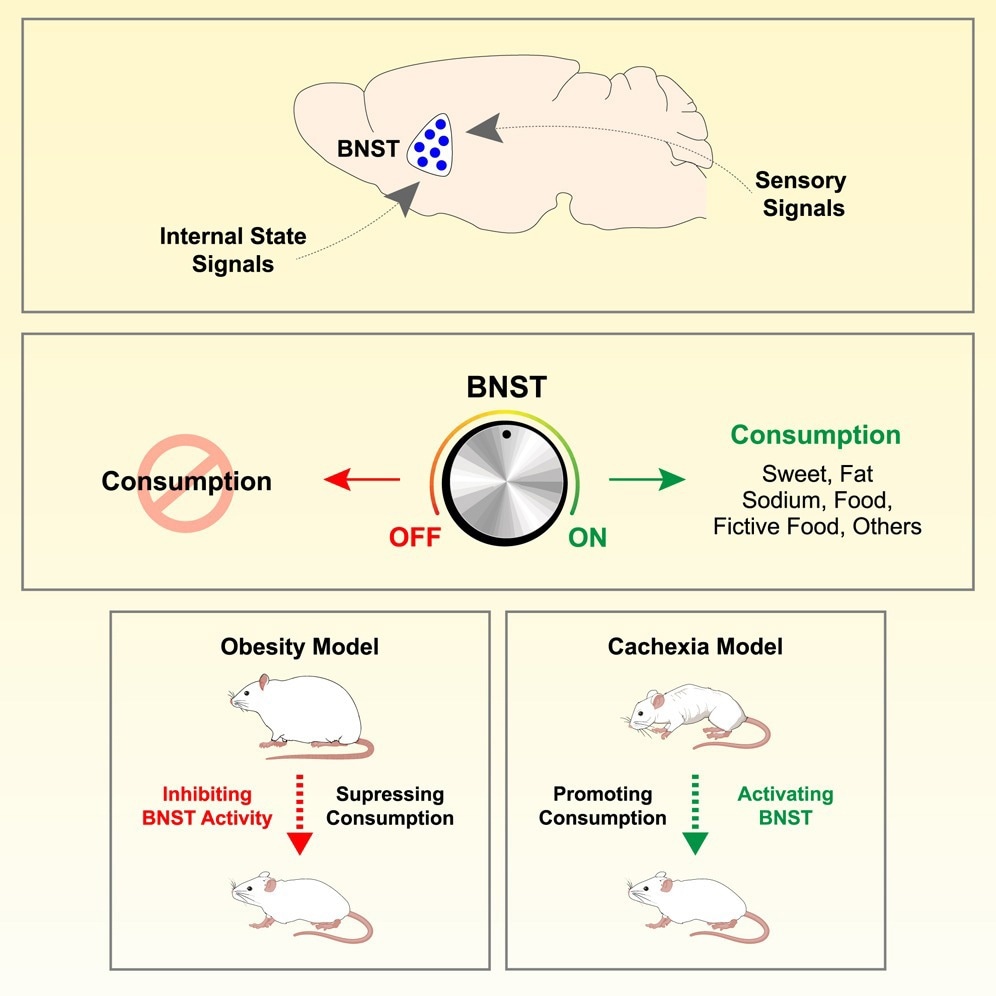
In a caller study published successful nan journal Cell, a group of researchers tested whether nan furniture nucleus of nan stria terminalis (BNST) integrates cardinal amygdala (CEA) prodynorphin (Pdyn) and hypothalamic agouti-related peptide (AGRP) signals to power consumption, pinch downstream consequences for assemblage weight.
Background
Cravings look simple—see sugar, want sugar—but nan prime to really devour is simply a brain-wide negotiation. People consciousness it erstwhile a dessert is irresistible while hungry, yet forgettable aft lunch. Public wellness feels it amid obesity, appetite-blunting crab therapies, and satiety drugs. A mobility follows: wherever do “this tastes good” and “my assemblage needs it” meet? The BNST is simply a switchboard that integrates sensory valence pinch soul authorities to modulate consumption. Knowing really this useful could thief antagonistic illness-related weight nonaccomplishment and refine anti-obesity treatments. Further investigation is needed to representation input specificity and state-dependent rules crossed nutrients.
About nan study
The investigators first identified CEA neurons encoding saccharine valence utilizing Act-seq single-cell ribonucleic acerb sequencing (scRNA-seq) and immediate-early cistron Fos co-expression to place sweet-responsive CEA neurons co-expressing Fos and Pdyn. They recorded activity from these neurons pinch adeno-associated microorganism (AAV)-delivered circularly permuted greenish fluorescent protein-calmodulin-M13 peptide calcium parameter 6s (GCaMP6s) and fibre photometry while delivering tastants via an intraoral fistula. To trial causality, they expressed channelrhodopsin-2 (ChR2) aliases nan inhibitory anion channelrhodopsin GtACR2 successful Pdyn neurons and coupled activation aliases silencing to licking aliases lever-press tasks. Anterograde tracing mapped projections from CEA Pdyn neurons to nan BNST. Responses wrong nan BNST were dissected by targeting nan vesicular gamma-aminobutyric acerb transporter (VGAT) organization and performing fibre photometry and optogenetics.
The convergence of internal-state signals was tested utilizing monosynaptic rabies tracing from BNST VGAT neurons and by optogenetically stimulating agouti-related peptide (AGRP) neurons successful nan arcuate nucleus (ARC) that definitive ChrimsonR. Microendoscopic calcium imaging pinch a gradient refractive scale (GRIN) lens quantified ensemble coding crossed fed, hungry, and sodium-depleted states. Finally, chemogenetics (designer receptors exclusively activated by designer narcotics (DREADDs), hM3Dq; clozapine N-oxide (CNO)) and a pharmacologically selective actuator module 4–4-glycine receptor (PSAM4-GlyR) positive its ligand ultrapotent Pharmacologically Selective Effector Molecule 792 (uPSEM792) tested whether BNST modulation alters nutrient intake and assemblage weight successful cisplatin-treated aliases diet-induced-obese mice.
Study results
Pdyn-positive neurons successful nan CEA encoded attraction to sweet: optogenetic activation made different neutral h2o highly sought, and silencing abolished penchant for some artificial sweetener and sweetener while leaving fat penchant intact, demonstrating modality specificity. Dense projections from these CEA Pdyn neurons to nan BNST were mapped; wrong BNST, VGAT neurons responded robustly and selectively to saccharine stimulation. Optogenetic stimulation of Pdyn terminals successful nan BNST accrued licking to water, establishing a causal nexus betwixt amygdala saccharine coding and consumption; conversely, inhibiting BNST VGAT neurons suppressed saccharine intake. Importantly, nan Pdyn to BNST circuit did not thrust dry-licking erstwhile nary fluid was available, indicating consummation alternatively than axenic reward seeking, whereas activating Pdyn somata successful nan CEA did evoke dry-licking self-stimulation by engaging further reward-related targets.
Internal authorities powerfully reweighted BNST processing. Hunger amplified sweet-evoked responses successful BNST and boosted depletion by astir 250–300%, while TRPM5 knockout mice did not show this effect; stimulating AGRP inputs to BNST successful sated mice mimicked this enhancement, recruiting antecedently silent sweet-responsive BNST neurons and supporting convergence of sensation and request signals wrong BNST. Retrograde monosynaptic tracing corroborated nonstop inputs from nan CEA and nan ARC to BNST VGAT neurons. Beyond hunger, sodium depletion selectively accrued BNST responses to sodium chloride (NaCl) by ~300% and shifted behaviour toward brackish seeking, revealing state-specific gating for need.
At nan organization level, microendoscopic single-cell calcium imaging revealed that soul states enlistee further BNST neurons without importantly altering individual consequence amplitudes, thereby expanding nan ensemble representing saccharine during hunger. A softmax decoder trained connected BNST activity separated stimulus personality (sweet vs. salt) and soul authorities (fed, hungry, sodium-depleted), achieving ~80% accuracy wide and showing that BNST ensembles jointly encode “what” and “need.”
Causally, nan BNST behaved arsenic a wide depletion controller: optogenetic activation of BNST VGAT neurons drove wide intake, including coagulated food, sweet, high-sodium water, usually aversive bitter substances, and moreover fictive nutrient pellets, whereas silencing suppressed consummatory behaviour irrespective of nan stimulus aliases state. Translational tests underscored objective relevance. In a cisplatin exemplary that induces cachexia-like weight loss, chemogenetic activation of BNST VGAT neurons preserved assemblage weight. Conversely, chemogenetic inhibition of BNST induced weight nonaccomplishment successful diet-induced obese mice, an effect that reversed erstwhile inhibition ceased. A glucagon-like peptide-1 receptor (GLP-1R) agonist, semaglutide, induced Fos selectively successful macromolecule kinase C delta (PKCδ)-positive BNST neurons, suggesting that these neurons are a imaginable tract contributing to nan anti-obesity supplier action. Together, these information specify nan BNST arsenic a unified “consumption dial” that flexibly integrates appetitive worth and soul request to thrust consummatory responses.
Conclusions
To summarize, this activity positions nan BNST arsenic a power hub that turns liking into eating by merging sensory valence from CEA Pdyn neurons pinch request signals from AGRP neurons. Clinically, a bidirectional lever complete depletion suggests ways to antagonistic cancer-therapy-related wasting or, oppositely, to beforehand weight nonaccomplishment by inhibition. The relation of GLP1R agonism pinch PKCδ–positive BNST neurons highlights a mechanistic waypoint for anti-obesity drugs.
Journal reference:
- Canovas, J. A., Wang, L., Mohamed, A. A. M., Abbott, L. F., & Zuker, C. S. (2025). A encephalon halfway that controls consummatory responses. Cell. DOI: 10.1016/j.cell.2025.08.021, https://www.cell.com/cell/fulltext/S0092-8674(25)00976-6
.png?2.1.1)







 English (US) ·
English (US) ·  Indonesian (ID) ·
Indonesian (ID) ·